Pharmaceutical companies face an expanding compliance burden driven by global regulations, complex supply chains, multi-party agreements, and rising expectations for transparency and audit readiness. Obligations are scattered across R&D, clinical trials, manufacturing, distribution, quality, safety, and vendor relationships. When these obligations are not clearly captured and monitored, compliance breaks.
The Problem: Fragmented contract repositories, inconsistent obligation tracking, siloed teams, and rapidly changing regulatory requirements from the Food and Drug Administration and the European Medicines Agency.
The Impact: Audit findings, penalties, supply delays, quality deviations, data governance issues, and significant financial exposure.
The Solution: Centralized contract visibility, AI-driven obligation extraction, automated workflows, and continuous monitoring supported by modern compliance orchestration platforms.
Is Your Pharma Compliance Framework Keeping Up With Regulatory Demands?
See how modern systems help pharmaceutical organizations centralize obligations, enforce accountability, and reduce compliance risk.
Why Contract Compliance Pressure Is Increasing in Pharma
Pharmaceutical companies operate in one of the most regulated industries worldwide. Every stage of the value chain is controlled by strict agreements: clinical trial contracts, quality agreements, CDMO and CRO partnerships, manufacturing and supply contracts, licensing and distribution agreements, data processing addenda, and safety reporting obligations.
The challenge is that the obligations buried inside these documents are not only detailed but also fluid. Regulatory expectations from agencies like the FDA, EMA, ICH, and national health authorities evolve regularly. New updates, changing guidelines, and multi-country regulatory submissions increase the complexity of managing contract-driven obligations.
Most companies still rely on manual processes, shared drives, or siloed tracking mechanisms. As a result, critical compliance requirements slip through unnoticed until an audit, inspection, or operational disruption reveals the gap.
Why Contract Compliance Fails in the Pharmaceutical Industry
Contract compliance fails because obligations remain unstructured, unassigned, and unmonitored across functions.
A. Complex, Multi-Layered Agreements
Clinical trial agreements, safety reporting obligations, data-sharing rules, GMP-driven quality commitments, and CDMO contracts contain thousands of individual obligations. These obligations influence regulatory submissions, manufacturing quality, product safety, and global distribution. When no system extracts and structures these details, compliance becomes guesswork.
B. Fragmented Storage Across Departments
Legal, regulatory, quality assurance, supply chain, and procurement store their agreements separately. This decentralization makes it nearly impossible for anyone to see a consolidated view of obligations across the lifecycle.
C. Rapidly Changing Regulations
The regulatory environment changes frequently. FDA’s evolving guidance, EMA’s updates to PV and manufacturing rules, and increasing scrutiny around data governance create obligations that must be implemented quickly. Most contracts and internal workflows lag behind these changes.
D. Supply Chain Complexity
Outsourced manufacturing, third-party logistics, raw material suppliers, and cross-border distribution channels introduce contract requirements that affect multiple partners. Without structured tracking, obligations go unmonitored.
E. Manual Processes and Siloed Ownership
Teams rely on spreadsheets, emails, and static trackers. Obligations are misinterpreted, miscommunicated, or executed inconsistently. This creates recurring compliance gaps across the organization.
Common Contract Compliance Gaps in Pharma in North America
Pharmaceutical companies in North America mostly struggle with compliance gaps linked to FDA-driven expectations, vendor oversight, and large-scale supply operations.

A. GMP and Quality Agreement Deviations
Quality Agreements outline manufacturing responsibilities, testing requirements, CAPA timelines, and documentation standards. When these obligations are untracked, organizations face deviations and audit observations.
B. Incomplete Vendor and Supplier Compliance Tracking
CDMOs, packaging vendors, raw material suppliers, and distributors must adhere to contractual requirements. Missed testing cycles, incomplete batch documentation, or delayed responses cause significant compliance issues.
C. Untracked Data-Sharing and Privacy Obligations
Agreements containing HIPAA, patient data, and PHI management responsibilities often lack ongoing monitoring, creating privacy risks.
D. Pricing, Rebates, and Market Access Compliance
Contractual pricing terms, rebate schedules, and market access requirements are prone to inconsistencies that lead to financial exposure.
E. Inadequate Pharmacovigilance Reporting Compliance
PV agreements require strict timelines for adverse event reporting. Manual tracking often results in late or incomplete submissions.
Common Contract Compliance Gaps in Pharma in Europe
European compliance gaps arise mainly due to stricter regulatory requirements, documentation-heavy obligations, and complex cross-border supply operations.
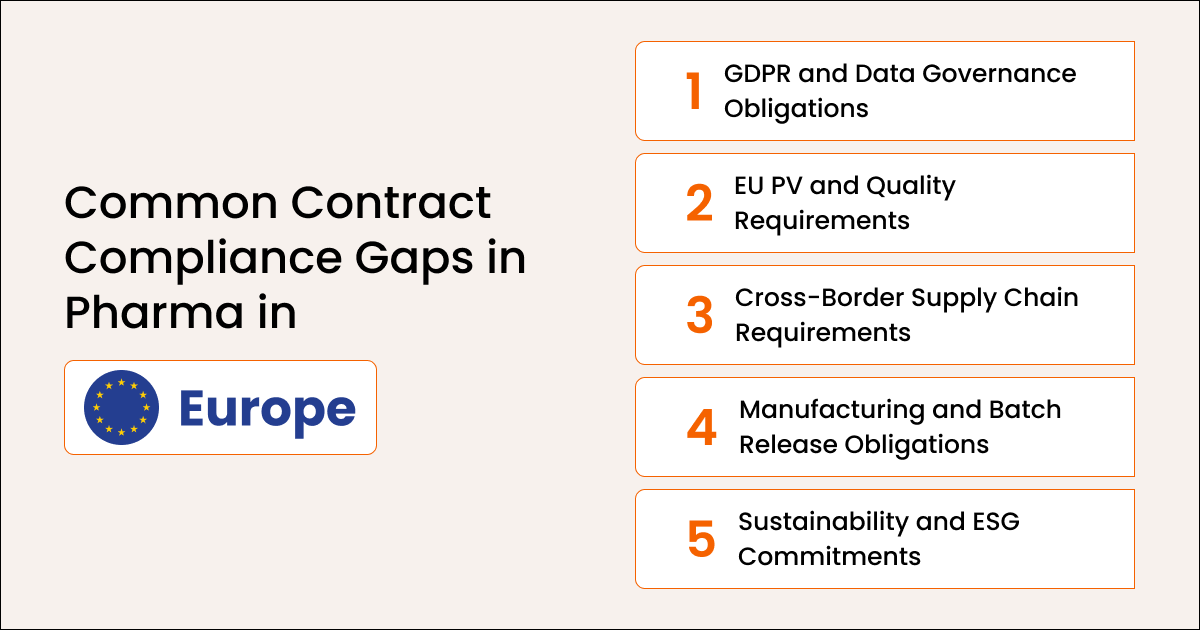
A. GDPR and Data Governance Obligations
Vendor and partner contracts often omit mandatory GDPR clauses or fail to provide adequate oversight of data processing activities, thereby increasing exposure.
B. EU PV and Quality Requirements
PV obligations, QPPV responsibilities, manufacturing controls, and batch release documentation demand structured execution that many teams cannot maintain manually.
C. Cross-Border Supply Chain Requirements
Contracts governing EU-wide distribution require precise documentation, serialization tracking, and reporting, creating multiple points for failure.
D. Manufacturing and Batch Release Obligations
Qualified Person (QP) responsibilities require strict adherence. Missing documentation or incorrectly tracked requirements often result in compliance findings.
E. Sustainability and ESG Commitments
Supplier and partner ESG clauses, now mandatory under emerging EU sustainability requirements, frequently remain unmonitored.
Why These Gaps Matter: The Business and Regulatory Impact
Compliance failures in pharma carry heavy consequences across quality, regulatory, financial, and operational dimensions.
A. Regulatory Penalties and Warning Letters
Missed obligations trigger warning letters, Form 483 observations, remediation requirements, and increased scrutiny from the FDA and EMA.
B. Supply Chain Delays and Product Shortages
Unmet contractual obligations can disrupt manufacturing or distribution, leading to delayed batches or product shortages.
C. Quality Failures and Safety Risk
Breakdowns in GMP compliance lead to deviations, contamination risks, or recalls that damage patient safety and brand trust.
D. Audit Failures and Remediation Costs
Missing documentation and inconsistent evidence extend audits and significantly increase remediation costs.
E. Financial Leakage and Partner Disputes
Failure to track pricing terms, rebates, penalties, or delivery commitments results in revenue loss and disputes with partners.
How to Detect Contract Compliance Gaps in Pharma
Detection begins with visibility, automation, and mapping contract language to operational workflows.
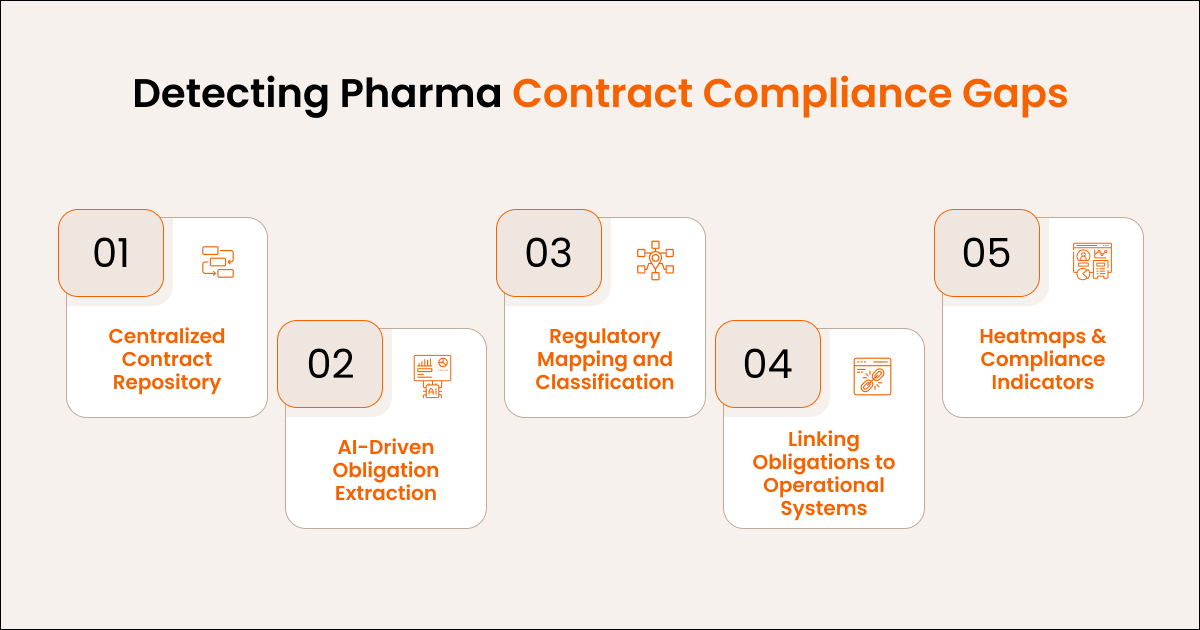
A. Centralized Contract Repository
Unifies Quality Agreements, licensing deals, CDMO contracts, clinical agreements, and distribution contracts in one access-controlled location.
B. AI-Driven Obligation Extraction
Automatically identifies GMP, PV, quality, supply chain, and commercial obligations hidden across large documents.
C. Regulatory Mapping and Classification
Links obligations to regulatory frameworks from the FDA, EMA, ICH, and others to ensure alignment and readiness for inspections.
D. Linking Obligations to Operational Systems
Connects extracted obligations to QMS, ERP, MES, and LIMS systems, turning legal language into actionable tasks.
E. Heatmaps and Compliance Indicators
Shows real-time insights into overdue obligations, high-risk areas, and upcoming regulatory deadlines.
Are Pharma Obligations Too Complex to Track Manually?
See how automated extraction and structured tracking simplify compliance for regulatory, quality, and supply-chain teams. Explore Aavenir’s Obligation Management Solution!
How to Bridge Compliance Gaps
Bridging gaps requires structure, cross-functional coordination, and standardized workflows.
- Standardize execution of Quality Agreements and PV obligations
- Automate assignment of contract tasks to functional owners
- Link deadlines to operational calendars and QMS workflows
- Implement vendor and supplier compliance dashboards
- Enforce SLA and KPI commitments consistently
- Set escalation paths for high-risk obligations
How to Continuously Monitor Contract Compliance
Monitoring contract obligations must be continuous, automated, and audit-ready.
- Real-time alerts for deadlines and deviations
- Exception reporting to flag compliance risks early
- Audit-ready logs for GMP, PV, and data-governance evidence
- Supplier compliance scorecards
- Batch-level and product-level monitoring tied to Quality Agreements
- Continuous risk scoring across data, quality, and supply chain
The Operational Answer: Staying Ahead of Obligations with Aavenir ComplianceNext
Modern pharma compliance requires a shift from manual oversight to structured, AI-driven governance.
Aavenir ComplianceNext provides a unified way for pharmaceutical companies to manage obligations at scale. It centralizes contracts, automatically extracts obligations, assigns responsibility to the right teams, tracks compliance, sends alerts, and maintains audit-ready evidence. It ensures every regulatory, quality, safety, and supply-chain requirement is visible, actionable, and monitored in real time.
ComplianceNext turns contract complexity into structured execution, supporting pharma organizations as they scale across geographies, product lines, and regulatory environments.
Conclusion
Pharmaceutical companies face increasing pressure from regulators, global supply chains, and complex multi-party agreements. Manual and siloed approaches leave organizations exposed to compliance gaps, audit risks, and operational disruptions. With AI-driven extraction, structured workflows, centralized visibility, and continuous monitoring, pharma companies can finally stay ahead of obligations and build a compliance framework that is accurate, proactive, and resilient.
Is Compliance Complexity Slowing Down Your Pharma Operations?
Discover how organizations are simplifying contract governance, improving visibility, and reducing risk through modern compliance automation.
FAQs
1. What makes contract compliance uniquely challenging in the pharmaceutical industry?
Pharma contracts involve highly regulated obligations tied to clinical trials, GMP manufacturing, quality agreements, safety reporting, data governance, and multi-country supply chains. With regulations from bodies like the Food and Drug Administration and European Medicines Agency changing regularly, obligations often become outdated, misaligned, or unmonitored, leading to recurring compliance gaps.
2. What types of obligations are commonly missed in pharma contracts?
Obligations related to adverse event reporting timelines, GMP and batch documentation requirements, QP responsibilities, data-sharing rules, supplier quality commitments, and distribution controls are frequently missed. These gaps often arise because obligations are buried deep within Quality Agreements, CDMO contracts, CRO agreements, and licensing deals with limited visibility across teams.
3. How do compliance gaps impact pharmaceutical companies?
Missed obligations can trigger regulatory findings, Form 483s, warning letters, delayed submissions, supply chain disruptions, product shortages, quality failures, and reputational impact. Financial consequences include pricing inaccuracies, rebate disputes, penalties, and remediation costs. In severe cases, unresolved gaps affect market access and patient safety.
4. Can AI really improve contract compliance in pharma?
Yes. AI can extract obligations from large documents, classify them into GMP, PV, safety, quality, or commercial categories, and map them to operational systems for execution. It can detect missed deadlines, deviations, or inconsistencies by comparing contract requirements with real-time data from QMS, ERP, MES, or LIMS systems. AI significantly reduces manual workload and improves inspection readiness.
5. How can pharmaceutical companies begin strengthening their contract compliance process?
Start by centralizing contracts into a unified repository and extracting obligations into a structured, trackable format. Then assign ownership, automate workflows, integrate obligations with quality and operational systems, and establish continuous monitoring for deadlines and deviations. A shift from reactive compliance to proactive governance is essential to staying audit-ready.