TL;DR: What’s Broken and How to Fix It
The pharmaceutical industry is governed by thousands of obligations across quality, safety, clinical operations, regulatory compliance, supply chain integrity, and commercialization. Most companies still track these obligations manually, which creates compliance gaps, manufacturing risks, supply chain exposure, audit issues, and revenue delays.
The solution is AI-powered obligation management that detects obligations within contracts and regulatory documents, assigns ownership, tracks execution, collects evidence, and prevents gaps.
Aavenir provides an end-to-end platform for detecting obligations, bridging operational gaps, and continuously monitoring compliance.
From Regulatory Burden to Operational Control
See how pharma companies convert obligation chaos into structured, audit-ready execution. Get the Aavenir Obligationflow Datasheet to learn more!
The High-Stakes Reality: Why Obligation Management Is Critical in the Pharmaceutical Industry
In pharmaceutical operations, obligation failures quickly escalate into quality issues, regulatory violations, or commercial delays.
The pharmaceutical industry operates in a highly controlled environment shaped by strict regulations, extensive documentation, global supply chains, and precise manufacturing protocols. Obligations come from GxP standards, regulatory filings, quality agreements, safety requirements, supplier contracts, CRO and CDMO relationships, and market access commitments.
Most obligations are locked inside lengthy PDFs, annexes, regulatory guidelines, or technical documents. With manual tracking, teams miss deadlines, overlook requirements, or lose visibility. These gaps show up as delayed submissions, deviations, rejected batches, recalls, or regulatory findings.
Pharma companies need a system that turns obligations into structured workflows with clear accountability.
Where Pharma Teams Struggle: A Breakdown of Obligation Gaps Across Stakeholders
Every team owns obligations but lacks a unified way to see and manage them. This creates fragmentation and hidden risk.
Regulatory and Quality Teams: Obligations Inside Dense Documentation
These teams handle obligations across ICH, FDA, EMA, MHRA, WHO, and GxP guidelines. Without automated extraction and mapping, compliance becomes reactive instead of controlled.
Manufacturing and Operations: Batch, Facility, and Traceability Complexity
Manufacturing teams manage equipment calibration, facility monitoring, sterility checks, and cleaning validation. When obligations are not tracked centrally, misses lead to deviations, quarantined batches, or slow batch release.
Supply Chain, CROs, CDMOs, CMOs: External Partner Obligations
Outsourcing partners carry obligations related to testing, transport, temperature control, data accuracy, safety reporting, and serialization. Manual tracking makes it difficult to enforce these commitments.
Commercial and Market Access: Complex Pricing and Reimbursement Rules
Pricing agreements, tenders, distributor contracts, and payer obligations vary by geography. Missed obligations result in pricing penalties or reimbursement disputes.
Legal and Contract Teams: Difficult Translation of Clause Language
Quality agreements, safety agreements, CTAs, MSAs, and licensing contracts contain operationally heavy obligations. Mapping them into tasks takes hours and often leads to inconsistencies.
Risk, Compliance, and Audit: High Evidence Burden
Pharma audits require complete evidence across manufacturing, supply chain, clinical, and quality operations. Manual systems create blind spots and slow down audit readiness.
Strengthen Compliance Across Regulatory and Audit Frameworks
Aavenir ComplianceNext centralizes regulatory obligations, automates evidence capture, and enables instant audit readiness for FDA, EMA, MHRA, GxP, and regional health agencies.
Obligation Management Challenges in North America
North American pharmaceutical companies face strict and evidence-heavy obligations that manual systems cannot sustain.
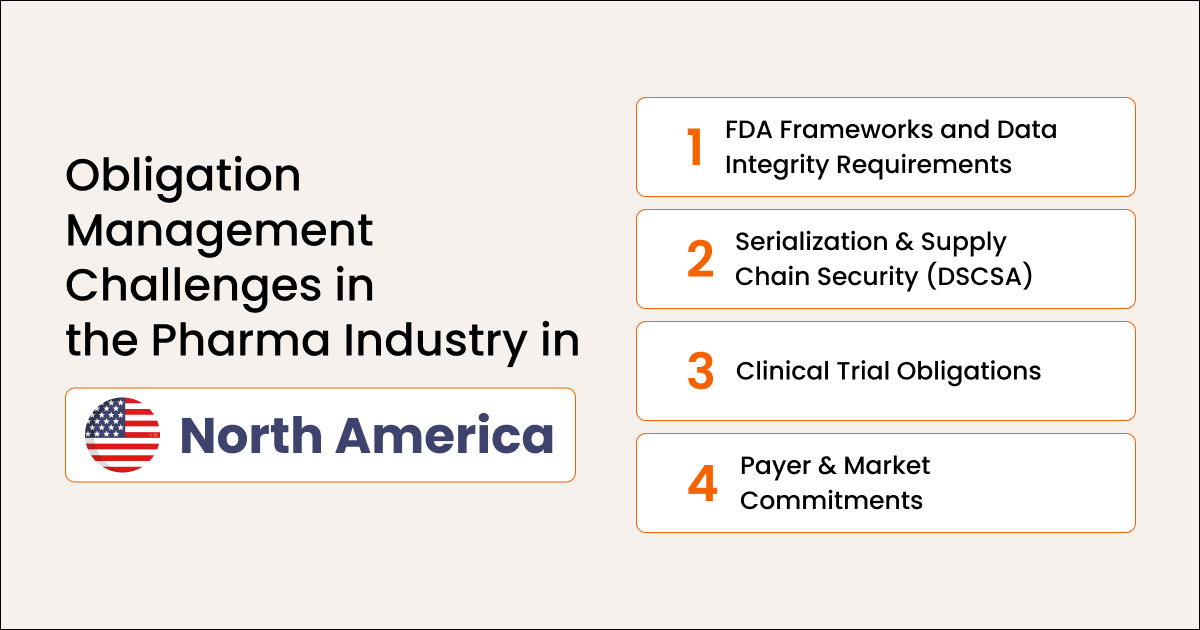
FDA: 21 CFR Requirements and Post-Market Obligations
Obligations span manufacturing documentation, electronic records, safety reporting, pharmacovigilance, and quality oversight. All require strong traceability. These obligations also evolve frequently, increasing the burden on teams to stay up to date and maintain continuous compliance.
DSCSA and Supply Chain Security
Serialization, wholesaler verification, and traceability obligations add complexity across large distribution networks. Failure to comply can disrupt product movement, impact patient access, and trigger regulatory intervention.
Clinical Trial and Research Obligations
Adverse event reporting, patient documentation, data accuracy, and protocol adherence add to operational pressure. Trial teams must also ensure real-time coordination with CROs, sites, and sponsors to avoid protocol deviations.
Market Access and Price Reporting Variability
Pricing, reimbursement, and reporting obligations differ across states and contracts, creating fragmentation without centralized control. Missed or inaccurate submissions can result in penalties, delayed reimbursement, or market withdrawal.
Obligation Management Challenges Across EMA and National Regulators
European pharmaceutical obligations shift frequently and require precise, cross-border coordination.

EU GMP and GDP Requirements
Batch documentation, labeling, release controls, and distribution obligations require close alignment across multiple teams, and even a small oversight can delay batch release or compromise compliance readiness during inspections.
EudraVigilance and Safety Reporting
Safety reporting has strict rules, timelines, and submission formats, and organizations must maintain continuous monitoring to ensure no adverse event or signal detection requirement is overlooked.
MDR and IVDR Obligations for Combination Products
Companies that produce combination products must meet additional documentation and quality requirements, which adds complexity because both device and drug obligations must be harmonized with regulatory expectations.
Cross-Border Release and Quality Requirements
Qualified Person obligations, batch certification rules, and country-specific release expectations make tracking challenging, mainly when multiple markets with unique regulatory timelines depend on synchronized release decisions.
What Happens When Obligation Management Fails in the Pharmaceutical Industry
Obligation gaps create downstream failures in safety, quality, compliance, and revenue.
- Quality failures lead to deviations, batch rejections, or recalls
- Missing documentation triggers FDA or EMA findings
- Supplier non-compliance disrupts manufacturing or cold-chain workflows
- Delayed submissions postpone product launches and reduce market access
- Audit findings result in remediation costs and reputational impact
- Inconsistent tracking slows down global operations
Manual tracking simply cannot keep up with the complexity of the pharmaceutical industry.
The AI Advantage: How Modern Technology Solves Pharma’s Obligation Challenges
AI converts hidden obligations into structured, measurable, and auditable workflows that pharmaceutical organizations can rely on for quality, regulatory, and operational continuity.
AI helps teams move from reactive compliance to proactive governance by revealing obligations buried in technical, regulatory, and contractual documents, ensuring they are owned, tracked, and completed with full traceability. This reduces the risk of deviations, delays, and audit findings while improving collaboration across global teams who depend on consistent execution.
AI-Powered Obligation Extraction
AI identifies obligations in regulatory documents, quality agreements, technical files, SOPs, and supplier contracts, transforming dense, complex text into clear, structured tasks that can be acted on immediately. This reduces manual interpretation effort and ensures that no regulatory, CRO, CDMO, or internal SOP requirements are missed during execution.
Automated Assignment and Routing
AI ensures obligations reach the correct teams across QA, manufacturing, clinical, compliance, and supply chain functions by assigning ownership, mapping timelines, and automatically triggering reminders. This eliminates ambiguous responsibility, reduces handoff errors, and ensures every obligation has a clear owner from day one.
Predictive Compliance Alerts
AI highlights obligations at risk of delay or non-performance by analyzing historical patterns, workload capacity, supplier performance, and regulatory timelines. This provides early warning signals that allow teams to intervene proactively, reducing the likelihood of deviations, missed submissions, or non-compliance events.
Centralized Evidence Management
AI-supported systems store documents, logs, approvals, batch records, calibration reports, and quality evidence in one location, ensuring instant audit readiness. This eliminates the scramble for documentation during inspections and creates a complete, chronological record that can withstand FDA, EMA, MHRA, and internal QA scrutiny.
Cross-Region Harmonization
AI standardizes obligations across FDA, EMA, MHRA, ICH, and GxP frameworks to reduce operational fragmentation and ensure global consistency. This is especially critical for companies operating across multiple sites and markets, where obligations differ subtly by region yet must be executed with uniform precision.
How Aavenir’s AI-Powered Obligation Management Solution Supports the Pharmaceutical Industry
Aavenir replaces fragmented obligation tracking with a single operational compliance system built for the pharmaceutical industry.
Aavenir Obligationflow, an AI-Powered Obligation Management Solution, enables pharmaceutical organizations to detect obligations, assign ownership, track execution, ensure visibility, and maintain audit readiness across all quality, regulatory, supply chain, and clinical operations.
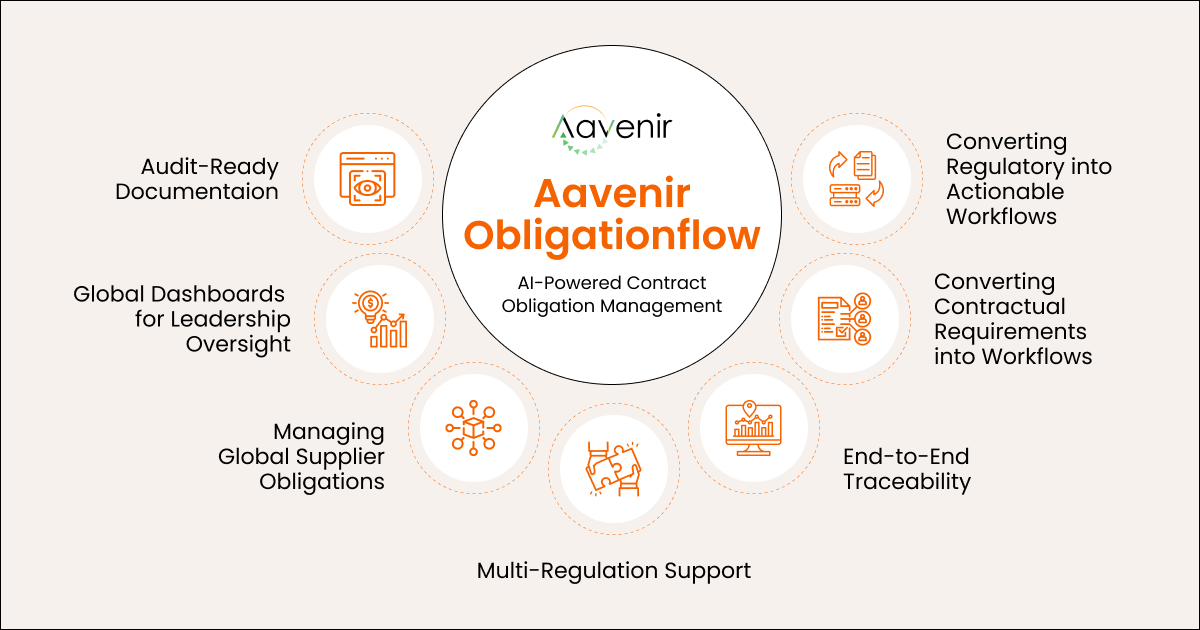
End-to-End Obligation Extraction
Aavenir extracts obligations from Quality Agreements, SOPs, CTAs, MSAs, and regulatory documents, turning dense text into clear, structured tasks. This ensures no requirement from regulators, partners, or internal teams is missed during execution.
Cross-Functional Workflow Management
The platform routes obligations to QA, manufacturing, clinical, regulatory, and supply chain teams with built-in reminders and escalations. This keeps work moving and prevents delays caused by unclear ownership.
Supplier and Outsourcing Partner Visibility
Aavenir centralizes obligations for CROs, CDMOs, CMOs, labs, and logistics partners in one place. This gives teams a clear view of partner performance and reduces the risk of missed commitments in outsourced operations.
Audit-Ready Documentation
All records, approvals, logs, and supporting evidence are linked directly to each obligation for instant audit readiness. This reduces preparation time and strengthens inspection confidence with FDA, EMA, and MHRA.
Leadership Dashboards for Global Visibility
Executives gain real-time visibility into risks, overdue items, partner performance, and site-level compliance. This helps leadership intervene early and improve decision-making across global operations.
Conclusion: Obligation Precision Is the Backbone of Pharma Success
In the pharmaceutical industry, obligation accuracy ensures safer products, stronger compliance, and faster market access.
The pharmaceutical industry faces increasing complexity across regulatory frameworks, global supply chains, safety reporting, and quality execution. Manual obligation tracking cannot keep pace with growing demand.
AI-powered obligation management provides the structure, visibility, and accuracy needed to reduce risk, accelerate execution, and maintain regulatory confidence.
Ready to see how AI removes obligation blind spots in pharmaceutical operations?
We can walk you through real examples, real workflows, and real results.
Frequently Asked Questions (FAQs)
1. Why is obligation management especially complex in the pharmaceutical industry?
The pharmaceutical industry operates under strict regulatory frameworks, including the FDA, EMA, MHRA, ICH, and GxP. Obligations span manufacturing, quality, safety, clinical trials, supply chain, and commercial agreements. These obligations are highly detailed, interdependent, and evidence-heavy. Manual tracking makes it difficult to maintain accuracy, meet deadlines, or satisfy audit expectations.
2. What types of obligations should pharma companies track?
Pharma organizations must track obligations across Quality Agreements, Safety Agreements, CRO and CDMO contracts, SOPs, regulatory filings, batch documentation, safety reporting timelines, serialization requirements, clinical protocols, and post-market commitments. Each comes with specific actions, deadlines, and evidence requirements.
3. How does poor obligation management affect compliance?
Gaps in obligation tracking lead to missed documentation, delayed reporting, incomplete quality records, and supplier non-compliance. These failures can result in regulatory findings, warning letters, product recalls, or loss of market authorization.
4. How can AI help pharmaceutical companies manage obligations more effectively?
AI can automatically extract obligations from regulatory documents and contracts, assign them to the right teams, track deadlines, and centralize evidence. It provides predictive alerts for at-risk obligations and ensures harmonized compliance across multiple geographies and regulatory bodies.
5. Why choose Aavenir for obligation management in the pharmaceutical industry?
Aavenir offers an AI-powered, end-to-end platform that detects, assigns, tracks, and audits obligations across quality, regulatory, manufacturing, supply chain, and clinical operations. It provides centralized visibility, automated workflows, supplier monitoring, and audit-ready documentation tailored to the pharmaceutical industry’s specific needs.